As we look ahead to next week and Thanksgiving, I have to say thank you to you for subscribing and reading. I got the stuffing knocked out of me this summer but knowing that this group of subscribers hangs in there and continues to read is nice. As I look ahead to the next year, let me know what you’d like to read more about. What is working. Or what you’d like more of.
FWIW. If someone is telling you that they have an idea of what will happen over the next four years in health policy, be a little skeptical. I mean usually there are big picture guesses for what could happen (focus on minority health, Medicaid, etc.) but there was no campaign promises on pharmaceutical health policy and, so far, the nominated leads (RFK Jr, Dr. Oz) don’t give a lot of direction. Mutterings about coding but … doesn’t seem like the thing? Pharma companies often have a list of the horrible things that could happen (based on their portfolios), time to pull that out and reevaluate with more, rather than less. Educated guesses are better than nothing in preparing for an unexpected Executive Order or Thursday afternoon proposed rule. If you need help with the list, let me know.
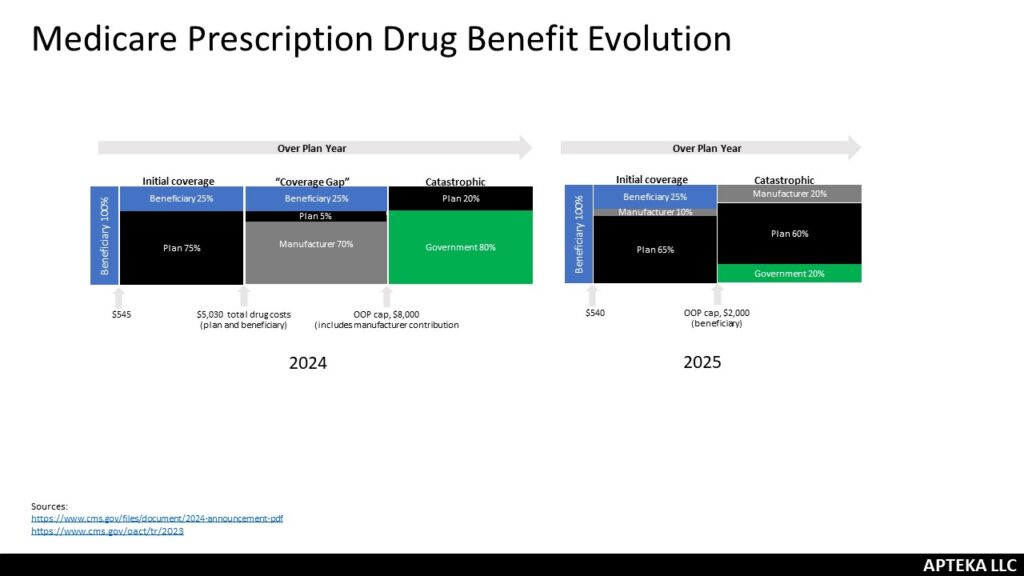
Reframing. Health Affairs Forefront had an article about the ways that Medicare causes patients to overpay for drugs. It highlighted siloed benefits, plan incentives for sick/healthy, coinsurance based on list price and the government liability change in Part D as part of the Inflation Reduction Act. It was a good reminder and reframing of the Part D design changes; while the out-of-pocket cap is a great change for beneficiaries, it was implemented in a way that costs patients more. The government went from paying 80% catastrophic to just 20%. The plans went from 15% to 60% and that will increase premiums which are paid by beneficiaries. Now, the government picks up the bulk of the monthly cost but still – benes will pay more. It is a pretty big jump and has consequences (hello demonstration plan). I appreciated the reminder said in a different way.
Updated. Kaiser Family Foundation updated their Medicare negotiation FAQs. Good starting place if you’re trying to remember what’s what.
Add to Future Case Study. Every day it feels like the media covers the price of a drug internationally versus what it costs in the United States. And the industry response is always a little disappointing because it is hard to explain that price has multiple meanings here. Usually it is U.S. list price versus their negotiated price. So apples and oranges. Another factor is that not all countries get the drugs we get in the U.S. – new case study – U.K. and Enhertu (for breast cancer.) They couldn’t come to an agreement on price.
